Batteries, as indispensable energy suppliers in our daily lives, are almost omnipresent. From simple remote controls to complex electronic devices, batteries play a crucial role. However, facing a dazzling array of battery products, a common doubt arises in many people’s minds: “Are all batteries alkaline?” To answer this question, we need to delve into the diversity and chemical principles of batteries.
The category of “alkaline batteries” refers specifically to a type of battery that uses alkaline electrolyte, typically potassium hydroxide, as the medium for the flow of electrical current between the cathode and anode. They are known for their long shelf life and are commonly used in many household devices.

Alkaline batteries, when compared to zinc chloride batteries or alkaline battery and non alkaline battery, are known for their long shelf life and are commonly used in many household devices.
Compared to non-alkaline batteries, alkaline batteries have slightly more energy and a longer lifespan. These household batteries are simple yet sophisticated, consisting of several main components.
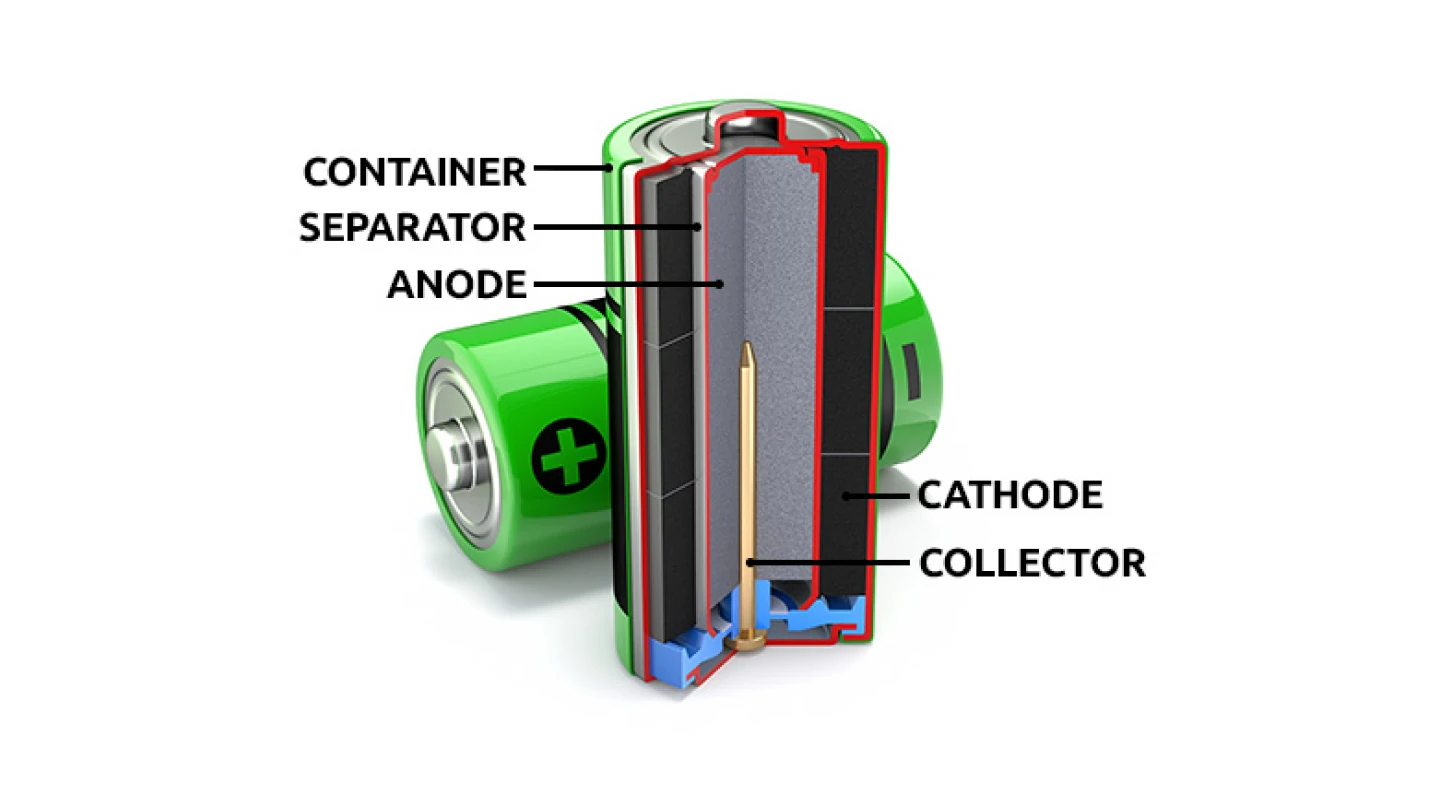
Container. The standardized steel structure holds the various components of the battery together.
Cathode. The part of the battery that generates the electric current.
Anode. The material, made from powdered zinc, is installed inside the container.
Separator. This material isolates the cathode from the anode, preventing a reaction unless the device is powered.
Electrolyte. It serves to contact the anode and aid in the flow of ions and electrons within the battery.
Collector. This is the brass pin located at the center of the anode, which collects the electric current and sends it to the negative terminal at the bottom of the battery.
What types of batteries are there?
There is a wide variety of batteries, each with different working principles and chemical compositions. Based on the type of electrolyte used, batteries can be classified into alkaline batteries and non-alkaline batteries.
1.Alkaline Batteries
Alkaline batteries, such as the common AA, AAA, C, and D cell batteries, use potassium hydroxide as the electrolyte and are known for their long service life and stable voltage output. These types of batteries are very popular in households and portable devices.
2.Non-Alkaline Batteries
Are all batteries alkaline? The answer is no. Here are some common types of non-alkaline batteries:
- Zinc-Carbon Batteries: These use ammonium chloride as the electrolyte and are relatively low-cost, but they do not have the energy density of alkaline batteries.
- Lithium Batteries: These use non-aqueous electrolytes, have a high energy density and long lifespan, and are widely used in modern electronic devices.
- Nickel-Metal Hydride (NiMH) Batteries: These are rechargeable batteries that use a potassium hydroxide solution as the electrolyte and are often used as an alternative to disposable alkaline batteries.
Alkaline Batteries And Non Alkaline
Alkaline Batteries
Alkaline batteries utilize potassium hydroxide as an alkaline electrolyte and are the most common household batteries, often used in devices such as remote controls, flashlights, and toys. They are favored by consumers for their higher energy output and longer service life. Their performance in low-temperature conditions is also relatively good, making them an ideal choice for outdoor activities and extreme environments.
Lithium Batteries
Lithium batteries use lithium ions as the anode and are commonly used in high-drain devices such as cameras, watches, and some portable electronic devices. Lithium batteries are characterized by their high energy density and long life, and they can operate stably in extreme temperatures and environments, offering more options for outdoor activities or extreme conditions.
Nickel-Cadmium (NiCd) Batteries
Nickel-cadmium batteries are rechargeable batteries that use nickel hydroxide and metallic cadmium as electrodes, commonly used in power tools, medical equipment, and some older electronic devices. They are known for their durability, high discharge rate, and good performance in low temperatures. However, they contain the toxic metal cadmium and have a memory effect, which reduces the battery capacity if not fully discharged before recharging.
Nickel-Metal Hydride (NiMH) Batteries
Nickel-metal hydride batteries are an efficient type of rechargeable battery that uses nickel as the positive electrode material and hydrogen-absorbing alloy as the negative electrode material, commonly used in consumer electronics, digital cameras, and hybrid vehicles. Compared to NiCd batteries, NiMH batteries have a higher energy density and greater capacity, are environmentally friendly and non-polluting, less prone to memory effects, and have a longer service life. However, compared to lithium-ion batteries, they have a shorter lifespan and a relatively higher self-discharge rate.
Lead-Acid Batteries
Lead-acid batteries use lead dioxide and sponge lead as electrodes and sulfuric acid as the electrolyte. They are commonly used in vehicles (as automotive batteries) and as backup power for uninterruptible power supplies (UPS). They have the advantages of high current, low cost, and safety and reliability, but due to their heavy weight, the presence of toxic lead, and relatively short service life, their range of use is limited and they are being phased out.
Zinc-Carbon Batteries
Zinc-carbon batteries use zinc and manganese dioxide as electrodes and an acidic electrolyte. They are typically used in low-drain devices such as remote controls, clocks, and flashlights. They have the advantages of being inexpensive and widely available, but compared to alkaline batteries, they have a lower energy density and shorter lifespan.
Silver Oxide Batteries
Silver oxide batteries use silver oxide as the cathode material and zinc as the anode. They are commonly used in small devices such as watches, hearing aids, and calculators, such as button cells made from silver oxide. They have the advantages of high energy density, stable voltage output, long life, and good low-temperature performance, but due to the high cost of silver, they are expensive.
Zinc-Air Batteries
Zinc-air batteries use the oxygen in the air as a reactant with zinc. They are commonly used in hearing aids and other small electronic devices. They have the advantages of high energy density and light weight, but they have a limited shelf life once exposed to air and are sensitive to environmental conditions.
Lithium-Ion (Li-ion) Batteries
Lithium-ion batteries are rechargeable batteries that use lithium compounds as cathode materials and are widely used in portable electronic devices such as smartphones, laptops, and electric vehicles. They have the advantages of high energy density, long life, and low self-discharge rate, but they are sensitive to high temperatures, and if damaged or improperly charged, they can overheat or explode, and they are relatively expensive.
Alkaline batteries Vs Non Alkaline
Although alkaline batteries perform well in many applications, in certain situations, non-alkaline batteries may be a better choice:
Lithium Batteries: For devices that require lightweight and high energy density, such as smartphones and laptops, lithium batteries are the preferred choice.
Nickel-Metal Hydride Batteries: For devices that require rechargeable batteries, nickel-metal hydride batteries offer an environmentally friendly and cost-effective solution.
Conclusion In summary, not all batteries are alkaline. The choice of battery should be based on usage requirements, performance needs, and environmental considerations. While alkaline batteries are an ideal choice in many situations, non-alkaline batteries also have their unique advantages in specific applications.
Understanding the different types of batteries and their characteristics can help us make wiser choices to meet our energy needs while considering cost and environmental impact. As technology advances, more innovative battery technologies may emerge in the future, providing more efficient and environmentally friendly energy solutions for our devices.
The Various Types of Alkaline Batteries
There are several types of alkaline batteries, including the commonly used AA AAA C D, and 9-volt batteries. According to a market analysis report by Grand View Research, these sized batteries are tailored to meet the energy requirements of different devices, from high-drain gadgets like digital camera to essential household items.
According to a market analysis report by Grand View Research, these sized batteries are tailored to meet the energy requirements of different devices, from heavy load gadgets like digital cameras to essential household items.
Other types of batteries include:
Nickel-Cadmium (NiCd): Rechargeable with high endurance for temperature extremes and a long cycle life but suffer from the memory effect and contain environmentally harmful cadmium.
Lithium-Ion (Li-ion): Known for high energy density, these rechargeable batteries power portable electronics and have become the standard for devices requiring long-lasting energy supply.
Nickel-Metal Hydride (NiMH): Offering higher capacity than NiCd without the toxic cadmium, used extensively in high-drain devices and hybrid vehicles.
Lead-Acid: The oldest type of rechargeable battery, characterized by the ability to deliver high surge currents, commonly used in vehicle starting systems.
Silver-Oxide: High energy-to-weight ratio, typically used in small-sized batteries for watches, calculators, and hearing aids.
Zinc-Carbon: Basic and affordable, with lower energy density, fitting for devices with low power requirements.
Lithium: Non-rechargeable with a long shelf life, found in critical applications like medical devices where battery replacement is challenging.

When one asks, “Are all batteries alkaline?” it is clear they are referring to a specific battery technology. Alkaline batteries are defined by their alkaline cell chemistry, which includes a combination of zinc and manganese dioxide, with an alkaline electrolyte typically made from potassium hydroxide. However, not all batteries are alkaline. According to data from the International Energy Agency, batteries come in various chemistries, including lithium-ion, nickel-cadmium, and lead-acid, each with applications based on their unique properties.
Exploring Alkaline Battery Chemistry
Alkaline battery chemistry is unique. The chemistry of alkaline batteries allows for high energy density and a long shelf life, which is why they are often preferred for many household devices. A report by the U.S. Environmental Protection Agency outlines that alkaline batteries can retain their charge for several years, distinguishing them from other battery types like nickel-cadmium or lead-acid, which have different discharge characteristics and applications.
What needs your attention?
Tips to remember:
Store them in a cool, dry place.
Remove them from electronic devices when not in use for extended periods.
Remove them from electronic devices powered by household alternating current (AC).
Keep them separate from metal objects like paper clips or coins that could affect the polarity of the batteries.
Do not use batteries of different brands or from different years together.
Uses of Alkaline Batteries
What are alkaline batteries used for? They are commonly found in applications that require reliable and consistent power over time, such as remote controls, wall clocks, and flashlights. The industry publication Battery Technology Today notes that the widespread use of AA and AAA alkaline batteries is a testament to their versatility and reliability in powering a broad range of devices.
Alkaline batteries are widely used for various everyday devices due to their long shelf life, reliable performance, and cost-effectiveness. Here are some common applications:
Household Devices
1. Remote Controls: For TVs, air conditioners, and other electronic devices.
2. Flashlights: Reliable power source for emergency lighting and outdoor activities.
3. Clocks and Watches: Maintaining accurate time over extended periods.
4. Toys: Powering children’s electronic toys and gadgets.
Portable Electronics
1. Radios: Ensuring consistent performance for portable and emergency radios.
2. Cameras: Providing reliable power for digital cameras and flash units.
3. Portable CD and MP3 Players: Extending the usage time of portable music players.
Health and Personal Care Devices
1. Electric Toothbrushes: Powering the motor for effective brushing.
2. Blood Pressure Monitors: Ensuring accurate readings in portable health devices.
3. Shavers and Trimmers: Providing cordless convenience for grooming tools.
Office Supplies
1. Wireless Mice and Keyboards: Offering long-lasting power for computer peripherals.
2. Calculators: Ensuring consistent performance in calculators used for work and study.
Household Gadgets
1. Thermostats: Powering programmable thermostats for home heating and cooling systems.
2. Smoke Detectors: Providing reliable power for essential safety devices.
3. Wireless Doorbells: Ensuring consistent operation for home doorbell systems.
Miscellaneous Uses
1. Game Controllers: Powering controllers for gaming consoles.
2. Portable Fans: Providing power for small, personal cooling devices.
3. LED Lights: Used in various battery-operated LED light fixtures.
alkaline batteries and non alkaline
In the field of battery technology, “alkaline batteries vs non alkaline” is a topic that is often discussed. Alkaline batteries and non-alkaline batteries have significant differences in many aspects, which affect their range of applications and the choices of users.
The debate over alkaline batteries versus non-alkaline batteries, such as alkaline batteries vs lithium batteries or alkaline batteries vs silver oxide batteries, usually revolves around the specific needs of the devices they power. For instance, according to a study by Power Source Magazine, lithium batteries are favored for high-drain devices due to their higher energy density and rechargeability. In contrast, alkaline batteries are often chosen for their long shelf life and stable voltage output in low-drain applications.
In the comparison of “alkaline batteries vs non alkaline,” there is no absolute winner, as the choice of which battery to use depends on specific application needs and personal preferences. Alkaline batteries have advantages in high energy output and longevity, while non-alkaline batteries are more attractive in terms of environmental friendliness, cost-effectiveness, and rechargeability. Understanding these differences helps users make informed choices based on their needs. As technology advances, we look forward to more innovative battery technologies emerging in the future to meet the growing energy demands and environmental requirements.
Read more: Alkaline vs non alkaline batteries




