what is a dry cell battery?
What is a dry battery cell and how does it differ from other battery types? The primary distinction lies in its construction and the nature of its electrolyte. Unlike wet cell batteries that contain a free-flowing liquid electrolyte, the electrolyte in a dry cell is immobilized as a paste. This setup not only minimizes the risk of leakage but also allows for versatile positioning and handling of the battery without the risk of spillage.
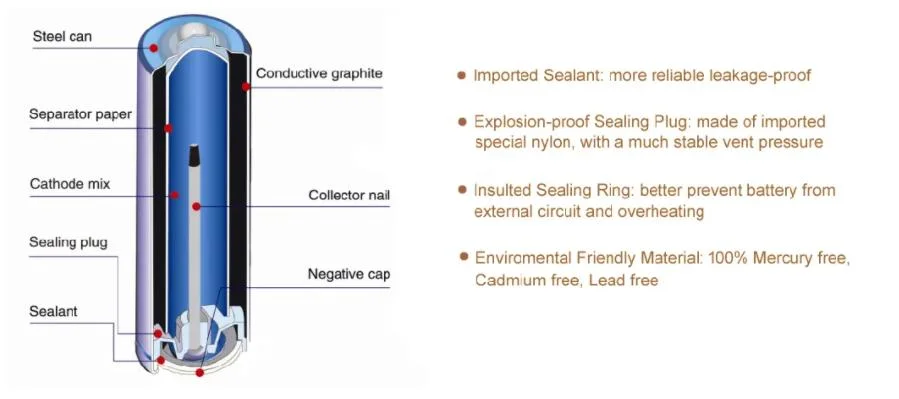
The typical dry cell consists of:
Zinc (Anode): This metal serves as both the container and the negative electrode.
Manganese Dioxide (Cathode): Acts as the positive electrode surrounded by a mix of carbon powder which improves conductivity.
Electrolyte Paste: Commonly comprising ammonium chloride mixed with zinc chloride, facilitating ion transfer necessary for the battery operations.
Key Components of a Dry Cell Battery:
Anode (Negative Electrode): Typically made from zinc, which also serves as the outer casing of the battery.
Cathode (Positive Electrode): Usually a carbon rod surrounded by a mixture of manganese dioxide and carbon powder. The manganese dioxide acts as a depolarizer, helping to prevent the buildup of gases within the cell.
Electrolyte: A moist paste that may include substances like ammonium chloride or zinc chloride. This paste facilitates the movement of ions within the battery, which are necessary for the chemical reactions that generate electricity.
A dry cell battery is a type of electrochemical cell where the electrolyte is immobilized as a paste, which prevents it from spilling or leaking. This characteristic makes dry cell batteries particularly useful for portable applications where liquid leakage could cause damage or be hazardous.
Read more: Why Choose DukeCell 9v Batteries?

How Does A Dry Cell Battery Work?
When the battery is connected to an external circuit, a chemical reaction occurs at the anode that releases electrons. These electrons travel through the external circuit to the cathode, providing the electrical energy needed to power electronic devices. Meanwhile, within the battery, ions move through the electrolyte from the anode to the cathode to balance the charge. This flow of electrons and ions continues as long as the chemical reaction can sustain it, which is until the reactants are depleted.
Advantages of Dry Cell Batteries:
Portability: Their solid or semi-solid electrolyte makes them less prone to leakage compared to wet cell batteries, which use a liquid electrolyte.
Safety: Dry cell batteries are safer to handle and transport because there’s no risk of spilling corrosive liquids.
Convenience: They are available in a variety of standard sizes (like AA, AAA, C, and D), making them easy to replace and dispose of.
Read more: Why Choose DukeCell 9v Batteries
How They Work and Their Advantages
Dry Cell Definition
Dry cells are batteries that use a paste-like electrolyte, in contrast to the liquid electrolyte found in wet cells. Due to the leak-proof characteristics and stability of dry cells, they have become the ideal choice for portable applications. The paste-like electrolyte allows for a compact and durable power source that can be seamlessly integrated into various devices. The term “dry cell” originates from the very low moisture content in the electrolyte, which reduces the risk of leakage and enhances safety. When discussing the Dry cell definition, we typically refer to batteries that use a solid or slightly moist paste-like electrolyte, in contrast to the liquid electrolyte of wet cells.
Dry cell definition emphasizes the state of their electrolyte, a design that makes dry cells safer to carry and use, reducing the risk of leakage. Additionally, the definition of dry cells also pertains to their structure, which consists of a positive electrode, a negative electrode, and an electrolyte. This structure Dry cell definition to generate electric current through chemical reactions.
In everyday life, whether it’s remote controls, flashlights, or children’s toys, the Dry cell definition sets our expectations and needs for portable power sources for these devices. In short, the definition of dry cells defines the fundamental characteristics of this type of battery, making it one of the indispensable energy sources in modern life.
How Does a Dry Cell Battery Work?
The operation of a dry cell battery involves a chemical reaction between the electrolyte paste and two electrodes: a zinc anode and a manganese dioxide cathode. Here’s how it works in detail:
Zinc Anode (Negative Terminal): Zinc undergoes oxidation, releasing electrons and zinc ions.
Manganese Dioxide Cathode (Positive Terminal): Manganese dioxide undergoes reduction, consuming electrons.
Electrolyte Paste: Typically, ammonium chloride or zinc chloride acts as the electrolyte, facilitating the flow of ions between the electrodes.
When the battery is connected to a device, electrons flow from the anode to the cathode through the external circuit, generating an electric current that powers the device. At Dukecell, we optimize these chemical reactions to ensure efficient and reliable energy output. For instance, our batteries can achieve an energy density of up to 300 watt-hours per kilogram (Wh/kg), significantly higher than the industry average.
Advantages of Dry Cell Batteries
Dry cell batteries offer several key advantages:
Portability:
Their compact design and leak-proof nature make them suitable for handheld and portable devices. The absence of liquid electrolytes reduces the weight, making them highly portable.
Long Shelf Life: Dry cells maintain their charge over longer periods, providing reliability when stored. Our dry cell batteries have a shelf life of up to 10 years, thanks to advanced sealing techniques and high-purity materials.
Durability:
They can operate in various orientations without the risk of spillage, enhancing their usability in different environments. They are also resistant to temperature variations, functioning efficiently between -20°C and 55°C.
Unique Selling Points of Dukecell Dry Cell Batteries
At Dukecell, we have refined our manufacturing processes to deliver superior dry cell batteries. Here are some unique features:
High Energy Density: Our batteries store a large amount of energy in a small volume, extending the operational time of devices. Our advanced cathode materials contribute to an energy density improvement of 20% over conventional dry cells.
Advanced Safety Mechanisms: We incorporate multiple safety features to prevent overheating and leakage, ensuring user safety. Thermal cut-off devices and pressure relief vents are standard in all our batteries.
Eco-Friendly Manufacturing: Our production processes minimize environmental impact, adhering to stringent sustainability standards. We use recyclable materials and have reduced hazardous substances in our batteries by 40%.
Custom Solutions: We provide tailored battery solutions to meet specific industrial and consumer needs, ensuring optimal performance. Our R&D team is equipped to design batteries that meet specific voltage, capacity, and size requirements, catering to niche applications.
Applications of Dry Cell Batteries
Dry cell batteries are used in a wide array of applications:
Consumer Electronics: Remote controls, flashlights, and handheld games. Our batteries provide consistent performance, ensuring your devices operate smoothly without frequent replacements.
Medical Devices: Portable medical equipment and emergency devices. Reliability is critical in medical applications, and our batteries are designed to deliver steady power over extended periods.
Industrial Tools: Instruments and tools requiring reliable power sources. Our batteries are engineered to withstand harsh industrial environments, providing dependable power for tools and equipment.
Dry Cell Battery Examples
Dry cell batteries come in various forms, each suited to different applications. Here are some common examples:
Alkaline Batteries: These are the most widely used dry cell batteries, found in devices such as remote controls, flashlights, and digital cameras. They offer a higher energy density and longer shelf life compared to other types.
Zinc-Carbon Batteries: Often used in low-drain devices like clocks and radios, these batteries are cost-effective and readily available.
Lithium Dry Cells: Used in high-performance applications like cameras and medical devices, lithium dry cells provide a higher energy output and longer lifespan.
Button Cell Batteries: These small, disc-shaped batteries power watches, hearing aids, and small electronic devices. They come in various chemistries, including silver oxide and alkaline.
At Dukecell, our range of dry cell batteries is designed to meet diverse needs, offering reliable performance across all these applications.
Contrast a Dry-Cell Battery with a Wet-Cell Battery
When contrast a dry-cell battery with a wet-cell battery, we first notice their construction differences. Dry-cell batteries, such as the common AAA and AA batteries, have solid electrodes and an electrolyte usually composed of a paste-like substance, which makes them easy to carry and use. In contrast, wet-cell batteries contain a liquid electrolyte, making them more suitable for applications in fixed locations.
Contrast a dry-cell battery with a wet-cell battery, we can also see that their maintenance requirements are quite different. Dry-cell batteries are sealed and do not require regular checks or replenishment of the electrolyte, whereas wet-cell batteries need regular maintenance to ensure the electrolyte levels are appropriate. Furthermore, when contrasting the discharge characteristics of dry-cell batteries with those of wet-cell batteries, we find that wet-cell batteries typically provide a more stable current output, but dry-cell batteries are more popular in mobile devices due to their portability.
In terms of energy density, contrast a dry-cell battery with a wet-cell battery, wet-cell batteries usually have a higher energy density, which means they can store more electrical energy in the same volume. However, due to their lower energy density, dry-cell batteries are generally lighter in weight and more suitable for lightweight portable devices. Overall, dry-cell and wet-cell batteries each have their advantages and limitations, and the choice between them depends on the needs of the specific application.
Understanding the differences between dry-cell and wet-cell batteries is crucial for selecting the right power source for your needs. Here’s a detailed comparison:
Electrolyte Composition:
Dry Cell: Utilizes a paste or gel electrolyte, making it leak-proof and suitable for portable devices.
Wet Cell: Contains a liquid electrolyte, typically sulfuric acid, which requires careful handling to prevent spills.
Portability:
Dry Cell: Compact and lightweight, making it ideal for handheld and portable applications.
Wet Cell: Heavier and bulkier due to the liquid electrolyte, commonly used in stationary applications like car batteries.
Maintenance:
Dry Cel: Maintenance-free, with a long shelf life and stable performance.
Wet Cell: Requires regular maintenance, including electrolyte level checks and periodic refilling.
Applications:
Dry Cell: Used in consumer electronics, medical devices, and small appliances.
Wet Cell: Predominantly found in automotive applications, backup power systems, and industrial equipment.
Durability:
Dry Cell: Resistant to leakage and can operate in various orientations without risk.
Wet Cell: More susceptible to leakage and requires a stable position to prevent electrolyte spills.
At Dukecell, we continuously innovate to enhance the performance and reliability of our dry cell batteries, ensuring they meet the highest standards of safety and efficiency.
Investigating:What are dry cell batteries used for?
Dry cell batteries, also known as disposable or primary batteries, are indispensable power sources in a wide range of devices due to their portability and ease of use. Here are some common uses for dry cell batteries:
Portable electronic devices: They are often used in devices that require a compact power source, such as digital cameras, remote controls, and MP3 players.
Toys and gadgets: Many toys, including remote-controlled cars and children’s electronic toys, rely on dry cell batteries to operate.
Flashlights: These batteries are frequently found in flashlights for emergency or everyday use.
Smoke detectors: Dry cell batteries are often used in home smoke detectors to ensure they function properly when needed.
Medical devices: Certain medical devices, such as blood pressure monitors and hearing aids, use dry cell batteries for convenience and ease of replacement.
Watches and clocks: Many watches and small clocks are powered by dry cell batteries, especially those that do not require frequent battery changes.
Outdoor and survival gear: Devices like headlamps, weather radios, and emergency beacons often use dry cell batteries for their reliability in various conditions.
Computer peripherals: Some computer mice, keyboards, and other peripherals are powered by dry cell batteries.
Entertainment devices: Devices like game controllers and portable gaming systems may use dry cell batteries.
Household appliances: Certain household appliances, such as cordless phones and electronic organizers, also rely on dry cell batteries.
The versatility of dry cell batteries makes them a staple in many homes and for on-the-go use, providing reliable power whenever and wherever it is needed.At Dukecell, our dry cell batteries are designed to meet your usage scenarios in daily life, offering exceptional performance, safety, and reliability.
A Dry Cell Battery Produces Energy: How Does A Dry Cell Work?
Understanding how a dry cell battery produces energy involves exploring the electrochemical reactions within the battery. Here’s a detailed explanation:
Electrolyte Composition: The electrolyte in a dry cell battery is a paste or gel, typically composed of ammonium chloride or zinc chloride. This electrolyte facilitates the movement of ions between the electrodes.
Electrodes:
Anode: Made of zinc, which undergoes oxidation, releasing electrons and zinc ions.
Cathode: Composed of manganese dioxide, which undergoes reduction, accepting electrons.
Chemical Reaction:
At the anode:
At the anode: Zn→Zn2++2e−Zn→Zn2++2e−
At the cathode: 2MnO2+2NH4++2e−→Mn2𝑂3+2NH3+H2𝑂2MnO2+2NH4++2e−→Mn2O3+2NH3+H2O
Electron Flow: Electrons flow from the anode to the cathode through the external circuit, generating an electric current that powers the connected device.
At Dukecell, we optimize these electrochemical processes to ensure our dry cell batteries deliver consistent and reliable energy. Our batteries achieve an energy density of up to 300 watt-hours per kilogram (Wh/kg), providing efficient power for various applications.




